Weight loss medications have been playing an increasingly important role in the field of long-term weight management. Especially the new generation of weight loss drugs not only demonstrate significant weight loss efficacy and good safety but also bring additional benefits such as metabolic improvement and heart-kidney health, opening up new paths for clinical treatment.
➤For most overweight or obese patients, especially those who have failed in weight loss in the past or cannot maintain weight loss, treatment with weight-loss drugs can be initiated directly if the indication is met.
➤For some patients, such as those with mild obesity (BMI 24~27.9 kg/m^2) and no obvious complications, weight-loss drug treatment can also be initiated when lifestyle interventions are ineffective (e.g., weight loss <5% in 3 months or below expectations).
What are the categories of weight loss drugs, their mechanisms of action, weight loss effects, and safety?
By category, this article takes stock of weight loss drugs that are currently on the market or about to be launched.
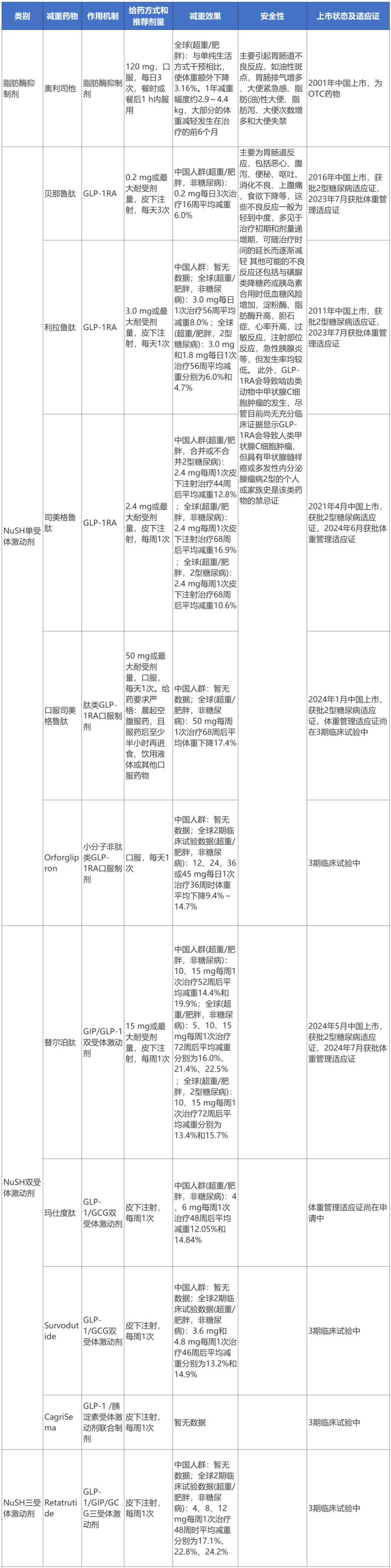
Table 1 Existing and upcoming weight loss drugs
(scroll up and down)
Note: OTC: Over-the-counter drugs; NuSH: Nutrient Stimulating Hormone; GLP-1: Glucagon-Like Peptide-1; GIP: Glucose-Dependent Insulinotropic Peptide; GCG: Glucagon
1. Lipase Inhibitors
The only lipase inhibitor currently on the market is Orlistat. This drug acts on the active sites of gastric lipase and pancreatic lipase, leading to the inactivation of lipase, thereby inhibiting the hydrolysis of triglycerides into absorbable free fatty acids and monoglycerides, which in turn affects the absorption of triglycerides and reduces calorie intake.
Orlistat was approved for marketing by the FDA in 1999 and in China in 2001, being the only non-prescription weight loss drug approved domestically. The recommended dose is 120 mg three times a day, to be taken with or within 1 hour after meals. It is recommended to apply this drug treatment on the basis of lifestyle and behavioral interventions.
In overweight or obese patients, Orlistat can slightly reduce weight, with an additional weight loss of 3.06% compared to lifestyle interventions alone. The weight loss range is approximately 2.9 to 4.4 kg per year, with most weight loss occurring in the first 6 months of treatment. The weight loss effect is related to the dosage of the drug, but there is no evidence to suggest that a dose greater than 120 mg three times daily has a greater weight loss effect. In addition to weight loss, Orlistat can also reduce the risk factors associated with obesity and the incidence of other obesity-related diseases or improve other diseases, including type 2 diabetes, insulin resistance, high cholesterol, high blood pressure, non-alcoholic fatty liver disease, and reduce fat content in organs. Long-term use of Orlistat can maintain treatment effects (including weight control and improvement of risk factors).
The main adverse reactions of Orlistat are gastrointestinal reactions related to the pharmacological action of blocking fat absorption, such as urgency for bowel movements, fatty/oily stools, oily discharge, increased frequency of bowel movements, fecal incontinence, oily spotting, increased gas with discharge, abdominal pain/discomfort, etc. In addition, there may be potential absorption disorders of fat-soluble vitamins (A, D, E, K) and certain medications (such as cyclosporine, thyroid hormones, antiepileptic drugs). Therefore, when using Orlistat for weight management, it is important to focus on the patient’s values and preferences, explain the adverse reactions and how to deal with them. Patient education should include dietary guidance, such as reducing the intake of high-fat foods as much as possible and regularly supplementing with multivitamins. It is also important to know if the patient is taking any of the aforementioned medications to avoid affecting the effectiveness of other diseases.
2. Nutrient Stimulating Hormone Receptor Agonists
1. NuSH Monoreceptor Agonists
The only NuSH monoreceptor agonists currently on the market in China are GLP-1RA and its analogs. GLP-1 is an incretin, which is secreted by L-cells in the small intestine under physiological conditions in response to nutrients in the gastrointestinal tract (such as carbohydrates, lipids, etc.). GLP-1 receptors are widely distributed in central and peripheral sites, including the hypothalamus, gastrointestinal tract, pancreatic islet cells, etc. Activation of GLP-1 receptors produces effects such as reducing appetite regulation in the hypothalamus, modulating the reward system to control eating behavior, and delaying gastric emptying, thereby exerting weight-loss effects. GLP-1RA simulates the effects of natural GLP-1 and exerts weight loss effects.
The GLP-1RA with weight management indications approved in China include the short-acting exenatide (approved in China in 2023, administered multiple times daily), the daily liraglutide (approved in China in 2023), and the weekly semaglutide (approved in China in 2024), all of which are subcutaneously injected formulations. Oral formulations of GLP-1RA (such as oral semaglutide, Orforglipron) are still in phase 3 clinical development and are planned to apply for weight management indications in China. Other GLP-1RA drugs such as dulaglutide, exenatide, lixisenatide, and luseogliflozin either do not have weight management indications or have not conducted weight loss-related clinical trials (only have type 2 diabetes indications), therefore, are not currently recommended for long-term weight management in overweight/obese patients.
➤Exenatide (injectable formulation): A short-acting GLP-1RA, the dose titration method for weight loss treatment is to start with a dose of 0.06 mg three times a day in the first week, increase the dose to 0.10 mg per dose in the second week, increase the dose to 0.14 mg per dose in the third week, increase the dose to 0.20 mg per dose in the fourth week, and maintain this dose for weight loss treatment. The recommended maintenance dose is 0.20 mg three times a day or the maximum tolerable dose. In phase 3 clinical trials conducted in the Chinese population of overweight/obese individuals (non-diabetics), the group treated with exenatide 0.2 mg three times a day showed an average weight loss of 6.0% over 16 weeks (significantly higher than the 2.4% in the placebo group).
➤Liraglutide (injectable formulation): A daily GLP-1RA, the dosing regimen for weight loss treatment involves starting with a dose of 0.6 mg once daily subcutaneously, increasing the daily dose by 0.6 mg per week, gradually escalating the daily dose to 3.0 mg or the maximum tolerable dose, and maintaining it. The global phase 3 clinical trials in the SCALE series showed that in overweight/obese individuals (non-diabetics), the group treated with liraglutide 3.0 mg once daily for 56 weeks had an average weight loss of 8.0% (significantly higher than the 2.6% in the placebo control group); in overweight/obese individuals with type 2 diabetes, the groups treated with liraglutide 3.0 mg and 1.8 mg once daily had average weight losses of 6.0% and 4.7% after 56 weeks (significantly higher than the 2.0% in the control group). As of now, results from phase 3 clinical trials with liraglutide in the Chinese population of overweight/obese individuals have not been disclosed.
➤Semaglutide (injectable formulation): A weekly GLP-1RA, the dosing regimen for weight loss treatment involves starting with a dose of 0.25 mg once every week subcutaneously for weeks 1 to 4, increasing the weekly dose to 0.5 mg for weeks 5 to 8, increasing the weekly dose to 1.0 mg for weeks 9 to 12, increasing the weekly dose to 1.7 mg for weeks 13 to 16, and increasing the weekly dose to 2.4 mg for week 17 onwards and maintaining it for weight loss treatment. The recommended maintenance dose is 2.4 mg once weekly or the maximum tolerable dose. In the global phase 3 clinical trials in the STEP series, overweight/obese individuals (non-diabetics) treated with semaglutide 2.4 mg once weekly subcutaneously for 68 weeks showed an average weight loss of 16.9% (2.4% in the placebo group); in overweight/obese individuals with type 2 diabetes, those treated with semaglutide 2.4 mg once weekly subcutaneously had an average weight loss of 10.6% after 68 weeks (3.1% in the placebo group). In the Chinese population of overweight/obese individuals (with or without type 2 diabetes), results from phase 3 clinical trials showed that after 44 weeks of treatment with semaglutide 2.4 mg once weekly subcutaneously, there was an average weight loss of 12.8% (significantly higher than the 3.0% in the placebo control group).
➤Semaglutide (oral formulation): A peptide type GLP-1RA oral formulation that utilizes sodium N-[(8S, 9S, 10R, 11S, 13S, 14S, 17S)-10,13-dimethyl-3-[(2R)-6-methylheptan-2-yl]-6,9,12,17-tetraoxo-14-(2-oxopyrrolidin-1-yl)-2,4,5,8,10,11,12,13,14,15,17,18,19,20,21-pentadecahydro-1H-cyclopenta[a]phenanthren-11-yl]hexanamide as an intestinal permeation enhancer, promoting absorption of semaglutide through the gastric mucosa into the bloodstream. Due to the very low oral bioavailability of this drug and very high variability in absorption/pharmacokinetics, there are strict dosing requirements, such as taking it on an empty stomach in the morning and waiting at least half an hour before eating, drinking liquids, or taking other oral medications. The dosing regimen for weight loss involves starting with a dose of 3 mg once daily for weeks 1 to 4, increasing the daily dose to 7 mg for weeks 5 to 8, increasing the daily dose to 14 mg for weeks 9 to 12, increasing the daily dose to 25 mg for weeks 13 to 16, and increasing the daily dose to 50 mg for week 17 onwards and maintaining it for weight loss treatment. Results from the global phase 3 clinical trials in the OASIS series showed that, in overweight/obese individuals (non-diabetics), treatment with oral semaglutide 50 mg once weekly for 68 weeks resulted in an average weight loss of 17.4% (1.8% in the placebo group). Clinical trials in the Chinese population of overweight/obese individuals with this drug are still ongoing.
➤Orforglipron (oral formulation): A small molecule non-peptide GLP-1RA oral formulation that is suitable for oral absorption, with eating and drinking liquids having no significant effect on its absorption. Results from the global phase 2 clinical trials showed that in overweight/obese individuals (non-diabetics), individuals treated with Orforglipron doses of 12, 24, 36, or 45 mg once daily for 26 weeks had average weight reductions of 8.6% to 12.6% (2.0% in the placebo group) and at 36 weeks had average weight reductions of 9.4% to 14.7% (2.3% in the placebo group). The drug is still in phase 3 clinical trials.
2. NuSH Dual Receptor Agonists
Increased understanding of NuSH and the significant success of single receptor agonist GLP-1RA in weight management have driven further research and development of weight loss drugs based on NuSH multi-receptor regulation to achieve better weight loss effects under long-term safe use. The existing NuSH dual receptor agonists are all injectable and weekly formulations, with only the GIP/GLP-1 dual receptor agonist tirzepatide approved for weight management indications in Europe and the US, while the GLP-1/GCG dual receptor agonist marizotide is in the process of applying for weight management indications; the GLP-1/GCG dual receptor agonist servitude and the GLP-1/amylin receptor agonist combination drug CagriSema are still in phase 3 clinical trials.
①GIP/GLP-1 dual receptor agonists: GIP and GLP-1 both belong to incretins, secreted by K-cells in the small intestine in response to nutrient stimulation in the gastrointestinal tract. Activation of the GIP receptor (GIP receptor, GIPR) not only produces some biological effects similar to GLP-1R activation (such as central appetite suppression, increased peripheral insulin sensitivity, etc.) but also acts on adipose tissue to regulate lipid storage and fat breakdown. The joint activation of GLP-1R and GIPR may produce unique synergistic effects on weight regulation through complex cooperative interactions. Tirzepatide is the first and currently the only GIP/GLP-1 dual receptor agonist, approved for long-term weight management in Europe and the US in 2023 and in China in 2024. Tirzepatide is a weekly formulation, with the dosing regimen involving a starting dose of 2.5 mg once weekly subcutaneously for weeks 1-4, followed by an increase of 2.5 mg every 4 weeks, gradually titrating to 15 mg or the maximum tolerable dose and maintaining it long-term. Results from the global phase 3 clinical trials in the SURMOUNT series showed that after 72 weeks of treatment with tirzepatide 5, 10, and 15 mg once weekly in overweight and obese patients (non-diabetic), the average weight reductions were 16.0%, 21.4%, and 22.5% respectively (2.4% reduction in the placebo control group); in patients with overweight/obesity and type 2 diabetes, after 72 weeks of treatment with tirzepatide 10 and 15 mg once weekly, the average weight reductions were 13.4% and 15.7% respectively (3.3% reduction in the placebo control group). In the Chinese population of overweight and obese patients (non-diabetic), after 52 weeks of treatment with tirzepatide 10 and 15 mg once weekly, the average weight reductions were 14.4% and 19.9% respectively (2.4% reduction in the placebo control group). Moreover, indirect comparative study results showed that compared to the mere activation of GLP-1R (semaglutide 2.4 mg), the weight loss effect of the GIP/GLP-1 dual receptor agonist (tirzepatide 15 mg) was more significant (average weight loss difference percentage of -5.92%).
②GLP-1/GCG dual receptor agonists: GCG is a peptide hormone synthesized and secreted by pancreatic α cells, which can directly act on the liver, promoting glycogen breakdown and gluconeogenesis, and can also promote fat breakdown and fatty acid oxidation by activating lipases, having a beneficial effect on energy expenditure. GLP-1/GCG can synergistically reduce food intake, increase energy expenditure, and GLP-1 can balance the blood sugar increase caused by GCG. Currently, marizotide is the only GLP-1/GCG dual receptor agonist in the weight management indication application stage. Results from the phase 3 clinical trials in the Chinese overweight and obese population (GLORY-1) showed that after 48 weeks of treatment with marizotide 4 and 6 mg once weekly, the average weight reductions were 12.05% and 14.84% respectively (0.47% reduction in the placebo control group). Additionally, another GLP-1/GCG dual receptor agonist servitude is in phase 3 clinical trials, with results from the global phase 2 clinical studies showing that after 46 weeks of treatment with servitude 3.6 and 4.8 mg once weekly, the average weight reductions were 13.2% and 14.9% respectively (2.8% reduction in the placebo control group).
③GLP-1/Amylin receptor agonist combination drug: Amylin is a peptide hormone secreted by pancreatic β cells, with physiological effects including regulating energy intake and food preferences, delaying gastric emptying, and harmoniously regulating blood sugar homeostasis, which includes GLP-1RA semaglutide-like acylated amylin. The combination drug CagriSema with semaglutide is expected to achieve stronger weight loss effects than semaglutide alone. Phase 3 clinical trials are currently ongoing.
3. NuSH Triple Receptor Agonists
There is currently only the GLP-1/GIP/GCG triple receptor agonist under study, with only retatrutide having entered phase 3 clinical trials. Global phase 2 clinical trials showed that in overweight/obese individuals (non-diabetics), retatrutide 4, 8, and 12 mg once weekly treatment for 48 weeks resulted in average weight reductions of 17.1%, 22.8%, and 24.2% respectively (2.1% reduction in the placebo group). Phase 3 clinical trials are currently ongoing.
Sources
Endocrine Society of the Chinese Medical Association. Guidelines for Long-term Weight Management and Clinical Application of Drugs for Obese Patients (2024). Chinese Journal of Endocrinology and Metabolism, 2024, 40(07): 545-564. DOI: 10.